
Grade 11 | Lesson 1
Dean of Students: dean@theschools.com
Tech Services: tech@theschools.com

Grade 11 | Lesson 1
Dean of Students: dean@theschools.com
Tech Services: tech@theschools.com
Science
Lesson Overview
Chemistry: The Science of Matter
• Composition, Structure, and Behavior
• Examining Matter: The Macroscopic View of Matter
• The Submicroscopic View of Matter
• Classification by Composition
• Mixed Matter
• The Separation of Mixtures into Pure Stuff
• Two Kinds of Mixtures
![]()
Chemistry: The Science of Matter
There's a tremendous variety of different stuff in the universe, and yet everything is the same in one way. It's all matter--from a supernova (large exploding star) in a galaxy 30 million light-years away to a piece of pepperoni on your pizza. How is a supernova different from a fresh-baked pizza? That's not too tough .... you can't call on the phone and order a supernova! But how is a supernova like a pizza? That's a question that chemistry answers. Chemistry is the science of asking and answering questions about stuff. Why does the cheese melt and a star explode? What makes the crust crisp and the star shine? Chemistry is the what, how, and why of stuff.
Composition, Structure, and Behavior
Chemistry is the science that investigates and explains the structure and properties of matter. Matter is the stuff that's all around you: the metal and plastic of a telephone, the paper and ink of a book, the glass and liquid of a bottle of soda, the air you breathe, and the materials that make up your body. A more formal definition of matter is anything that takes up space and has mass. Mass is the measure of the amount of matter that an object contains. On Earth, mass is usually equated with weight. What isn't matter? The heat and light from a lamp are not matter; neither are thoughts, ideas, radio waves, and magnetic fields.
The structure of matter refers to its composition--what matter is made of--as well as how matter is organized. The behavior of matter describes the characteristics or properties of matter, including the changes that matter undergoes.
You can determine some properties of a particular chunk of matter just by examining or manipulating it. What color is it? Is it a solid, liquid, or gas? If it's a solid, is it soft or hard? Does it burn? Does it dissolve in water? Does something happen when you mix it with another kind of matter? You determine all of these properties by examining and manipulating a chunk of stuff.
Although you can find out a lot about a piece of material just by looking at it and doing simple tests, you can't tell what something is made of only by looking at it, no matter whether it is a spoonful of sugar or a rock picked up by a robot on the surface of Mars. Measurements usually must be made or chemical changes observed.
Science is all around you. It is such a common part of your life that you probably take it for granted. Have you ever wondered why there are seasons, why volcanoes erupt, or whether life exists on other planets? Do you wonder how people find the answers to these and other questions?
Examining Matter: The Macroscopic View of Matter
Observations of the composition and behavior of matter are based on a macroscopic view. Matter that is large enough to be seen is called macroscopic, so all of your observations in chemistry, and everywhere else, start from this perspective. The macroscopic world is the one you touch, feel, smell, taste, and see.
In the same way, the appearance and properties of a piece of matter are the result of its structure. Although you may get hints of the actual structure from a macroscopic view, you must go to a submicroscopic perspective to understand how the hidden structure of matter influences its behavior.
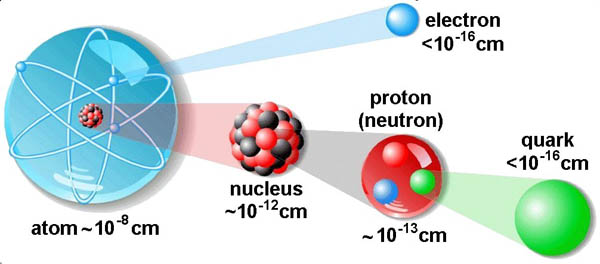
The Submicroscopic View of Matter
The submicroscopic view gives you a glimpse into the world of atoms. It is a world so small that you cannot see it even with the most powerful microscope, hence the term submicroscopic. You learned in earlier science courses that matter is made up of atoms. Compared with the macroscopic world, atoms are so small that if the period at the end of this sentence were made up of carbon atoms, it would be composed of more than
100 000 000 000 000 000 000 (100 quintillion) carbon atoms. If you could count all of those atoms at a rate of three per second, it would take you a trillion years to finish. Fortunately, in chemistry, you will spend your time becoming acquainted with atoms rather than counting them.
Classification by Composition
A powerful way to classify matter is by its composition. This is the broadest type of classification. When you examine an unknown piece of stuff you first ask, "What is it made of?" Sucrose is composed of the elements carbon, hydrogen, and oxygen. This is a qualitative expression of composition. A qualitative observation is one that can be made without measurement.
After a qualitative analysis, the next question that you might ask is how much of each of the substances is present. For sucrose, the answer to that question is that 100 g of sucrose contains 42.1 g of carbon, 51.4 g of oxygen, and 6.5 g of hydrogen. This is a quantitative expression of composition. A quantitative observation is one that uses measurement. You make quantitative measurements every day when you answer such questions as What's the temperature? How long was the pass? How much do you weigh? Pure substance or a mixture?
The most general way to classify matter by composition is in terms of purity. There are only two categories here. A sample of matter is either pure--made up of only one kind of matter--or it is a mixture of different kinds of matter. What does it mean to say that a chunk of matter is pure? In chemistry, pure means that every bit of the matter being examined is the same substance. A substance is matter with the same fixed composition and properties. A substance can be either an element or a compound. Any sample of pure matter is a substance.
If the sugar in a bag from the supermarket is a substance, then it is pure sucrose. Every bit of matter in the bag must have the same properties and the same fixed composition as every other bit. Now, consider a bag of high-quality, dry, white sand. White sand is the common name for a substance called silicon dioxide. It is white and crystalline like sugar, and, when the sand is examined, every particle has the same fixed composition (53.2 percent oxygen, 46.8 percent silicon). Both sand and sucrose are substances, but they have different properties and compositions.
Mixed Matter
Suppose you mix the pure white sand with the pure white sucrose. You may not be able to notice any difference, but if you add this mixture to your cereal or tea, it's a different story. The sweet taste of the sugar is still there, but so is the grittiness of the sand. Every particle of this stuff is not the same, so the properties are not the same throughout. Some parts taste sweet, while others are gritty and tasteless. The composition is also not fixed, but instead depends on how much sand is mixed with the sugar.
The Separation of Mixtures into Pure Stuff
One characteristic of a mixture is that it can be separated into its components by physical processes. The word physical means that the process does not change the chemical identity of a substance. How could you separate a sand/sugar mixture into pure sand and pure sucrose? The simplest physical means would be to look at it with a microscope and separate the bits of sugar and sand with tweezers. You are right in thinking that there must be an easier way.
Separating mixtures by using physical changes is one easier way. A physical change is a change in matter that does not involve a change in the identity of individual substances. Examples of physical changes include boiling, freezing, melting, evaporating, dissolving, and crystallizing. Separation of a mixture by physical changes takes advantage of the different physical properties of the mixed substances. Physical properties are characteristics that a sample of matter exhibits without any change in its identity. Examples of the physical properties of a chunk of matter include its solubility, melting point, boiling point, color, density, electrical conductivity, and physical state (solid, liquid, or gas).
Two Kinds of Mixtures
Sometimes, when you look at a sample of matter, it's easy to tell that the sample is a mixture. This kind of mixture is called a heterogeneous mixture. The prefix hetero means "different." A heterogeneous mixture is one with different compositions, depending upon where you look. The components of the mixture exist as distinct regions, often called phases. Orange juice and a piece of granite are examples of heterogeneous mixtures.
The separation of sand and sugar took advantage of a difference in the physical properties of the two substances. Sugar dissolves in water but sand does not. When sugar dissolves in water, the two pure substances, sugar and water, combine physically to form a mixture that has a constant composition throughout. This means that no matter where you sample the mixture, you find the same combination of sugar and water. Even with a powerful light microscope, you could not pick out a bit of pure sugar or a drop of pure water. This type of mixture is called a homogeneous mixture. The prefix homo means "the same." Homogeneous mixtures are the same throughout. Another name for a homogeneous mixture is solution. Even though solutions may appear to be one pure substance, their compositions can vary. For example, you could make your tea very sweet by dissolving a lot of sugar in it or less sweet by dissolving only a little bit of sugar.
When you hear the word solution, something dissolved in water probably comes to mind. But liquid solutions do not have to contain water. Gasoline is a liquid solution of several substances, but it contains no water. Some solutions are gases. Air, for example, is a homogeneous mixture of several gases. Some solutions are solid. Alloys are solid solutions that contain different metals and sometimes nonmetallic substances. Steel, for example, is a general term for a range of homogeneous mixtures of iron and substances such as carbon, chromium, manganese, nickel, and molybdenum. Because pure gold is soft and bends easily, most gold jewelry is not made from pure gold, but rather an alloy of gold with silver and copper.
When you dissolve sugar in water, sugar is the solute--the substance being dissolved. The substance that dissolves the solute, in this case water, is the solvent. When the solvent is water, the solution is called an aqueous solution. Many of the solutions you encounter are aqueous solutions, for example, soda, tea, contact-lens cleaner, and other clear cleaning liquids. In addition, most of the processes of life occur in aqueous solutions. The chemistry of the real world is mostly the chemistry of mixtures. Dig a hole, buy something at a grocery store, pick an apple from a tree, or take a deep breath. The stuff you dig, buy, pick, or inhale is a mixture. However, the behavior of mixtures is based on the composition, structure, and behavior of the pure substances that compose them.